| Symbol | Definition | Relevant Standard |
|---|---|---|
|
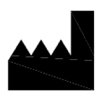
Manufacturer Indicates the medical device manufacturer. |
ISO 15223-1:2020. 5.1.1 ISO 7000 reg. no. 3082 |

|
Authorized representative in the European Community. Indicates the Authorized representative in the European Community. |
ISO 1552-1:2020. 5.1.2 |

|
Date of manufacture. Indicates the date when the medical device was manufactured. |
ISO 15223-1:2020. 5.1.3 ISO 7000 reg. no. 2497 2004-01-15 |

|
Use-by date. Indicates the date after which the medical device is not to be used. |
ISO 15223-1:2020. 5.1.4 ISO 7000 reg. no. 2607 2004-01-15 |

|
Batch code. Indicates the manufacturer's batch code so that the batch or lot can be identified. |
ISO 15223-1:2020. 5.1.5 ISO 7000 reg. no. 2492 2004-01-15 |

|
Catalogue number. Indicates the manufacturer's catalogue number so that the medical device can be identified. |
ISO 15223-1:2020. 5.1.6 ISO 7000 reg. no. 2493 2004-01-15 |

|
Serial number. Indicates the manufacturer's serial number so that a specific medical device can be identified. |
ISO 15223-1:2020. 5.1.7 ISO 7000 reg. no. 2498 2004-01-15 |

|
Importer. Indicates the entity importing the medical device into the locale. |
ISO 15223-1:2020. 5.1.8 ISO 7000-3725 2019-11-01 |
|
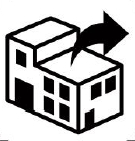
Distributor. Indicates the entity distributing the medical device into the locale. |
ISO 15223-1:2020. 5.1.9 ISO 7000-3724 2019-11-01 |

|
Country of Manufacture. To identify the country of manufacture of products. |
ISO 15223-1:2020. 5.1.11 ISO 7000-6049 2012-07-14 |

|
Sterile. Indicates a medical device that has been subjected to a sterilization process. |
ISO 15223-1:2020. 5.2.1 ISO 7000-2499 2004-01-15 |

|
Non-sterile. Indicates a medical device that has not been subjected to a sterilization process. |
ISO 15223-1:2020. 5.2.7 ISO 7000 reg. no. 2609 2004-01-15 |
|
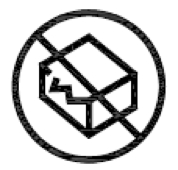
Do not use if package is damaged. Indicates a medical device that should not be used if the package has been damaged or opened. |
ISO 15223-1:2020. 5.2.8 ISO 7000 reg. no. 2606 2004-01-15 |
|
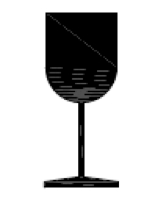
Fragile, handle with care. Indicates a medical device that can be broken or damaged if not handled carefully. |
ISO 15223-1:2020. 5.3.1 ISO 7000 reg. no. 0621 2014-06-04 |
|
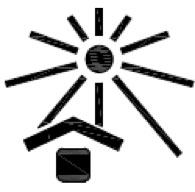
Keep away from sunlight. Indicates a medical device that needs protection from light sources. |
ISO 15223-1:2020. 5.3.2 ISO 7000 reg. no. 0624 2014-06-04 |
|
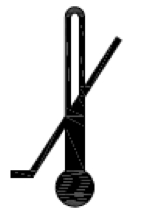
Lower limit of temperature. Indicates the lower limit of temperature to which the medical device can be safely exposed. |
ISO 15223-1:2020. 5.3.5 ISO 7000 reg. no. 0534 2004-01-15 |
|
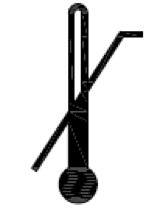
Upper limit of temperature. Indicates the upper limit of temperature to which the medical device can be safely exposed. |
ISO 15223-1:2020. 5.3.6 ISO 7000 reg. no. 0533 2004-01-15 |
|
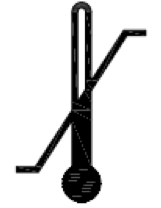
Temperature limit. Indicates the temperature limits to which the medical device can be safely exposed. |
ISO 15223-1:2020. 5.3.7 ISO 7000 reg. no. 0632 2014-06-04 |
|
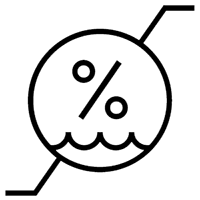
Humidity limitation. Indicates the range of humidity to which the medical device can be safely exposed. |
ISO 15223-1:2020. 5.3.8 ISO 7000 reg. no. 2620 |
|
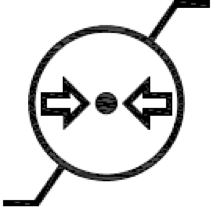
Atmospheric pressure limitation. Indicates the range of atmospheric pressure to which the medical device can be safely exposed. |
ISO 15223-1:2020. 5.3.9 ISO 7000 reg. no. 2621 2004-01-15 |

|
Biological risks. Indicates that there are potential biological risks associated with the medical device. |
ISO 15223-1:2020. 5.4.1 ISO 7000 reg. no. 2621 2004-01-15 |
|
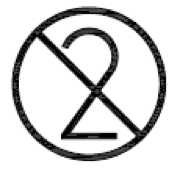
Do not re-use. Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure. |
ISO 15223-1:2020. 5.4.2 ISO 7000 reg. no. 2621 2004-01-15 |
|
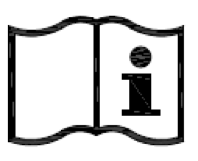
Consult instructions for use. Indicates the need for the user to consult the instructions for use. |
ISO 15223-1:2020. 5.4.3 ISO 7000 reg. no. 2621 2004-01-15 |
|
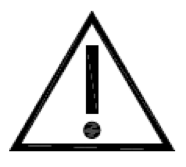
Caution. Indicates the need for the user to consult the instructions for use for important cautionary information such as warnings and precautions that cannot, for a variety of reasons, be presented on the medical device itself. |
ISO 15223-1:2020. 5.4.4 ISO 7000 reg. no. 0434A 2004-01-15 |

|
Contains or presence of natural rubber latex. Indicates the presence of natural rubber or dry natural rubber latex as a material of construction within the medical device or the packaging of a medical device. |
ISO 15223-1:2020. 5.4.5 ISO 7000, symbol 2725 2005-09-08 |

|
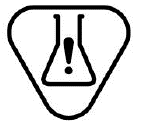
Contains a medicinal substance. Indicates a medical device that contains or incorporates a medicinal substance. |
ISO 15223-1:2020. 5.4.7 ISO 7000-3702 2019-10-18 |
|
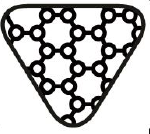
Contains Hazardous substances. Indicates a medical device that contains substances that can be carcinogenic, mutagenic, reprotoxic (CMR), or substances with endocrine disrupting properties |
ISO 15223-1:2020. 5.4.10 ISO 7000-3723 2019-11-01 |
|
Contains nano materials. Indicates a medical device that contains nano materials |
ISO 15223-1:2020. 5.4.7 ISO 7000-3702 2019-10-18 |
|
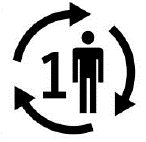
Single patient multiple use. Indicates a medical device that may be used multiple times (multiple procedures) on a single patient |
ISO 15223-1:2020. 5.4.12 ISO 7000-3706 2019-10-18 |
|
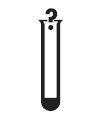
For IVD performance evaluation only. Indicates an IVD device that is intended to be used only for evaluating its performance characteristics before it is placed on the market for medical diagnostic use. |
ISO 15223-1:2020. 5.5.6 ISO 7000-3083 2011-10-03 |
|
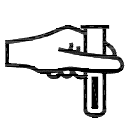
Sampling site. Indicates a medical device or blood processing application that includes a system dedicated to the collection of samples of a given substance stored in the medical device or blood container. |
ISO 15223-1:2020. 5.6.1 ISO 7000 reg. no. 2715 2005-09-08 |
|
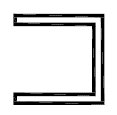
Fluid path. Indicates the presence of a fluid path. |
ISO 15223-1:2020. 5.6.2 SO 7000 reg. no. 2722 2005-09-08 |

|
Non-pyrogenic. Indicates a medical device that is non-pyrogenic. |
ISO 15223-1:2020. 5.6.3 SO 7000 reg. no. 2724 2005-09-08 |
|
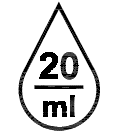
Drops per milliliter. Indicates the number of drops per millilitre. |
ISO 15223-1:2020. 5.6.4 SO 7000 reg. no. 2726 2005-09-08 |
|
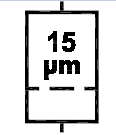
Liquid filter with pore size. Indicates an infusion or transfusion system of the medical device that contains a filter of a particular nominal pore size. |
ISO 15223-1:2020. 5.6.5 SO 7000 reg. no. 2727 2005-09-08 |
|
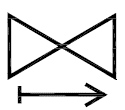
One-way valve Indicates a medical device with a valve that allows flow in only one direction. |
ISO 15223-1:2020. 5.6.6 SO 7000 reg. no. 2728 2005-09-08 |
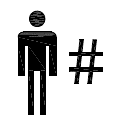
|
Patient number. Indicates a unique number associated with an individual patient. |
ISO 15223-1:2020. 5.7.1 SO 7000 reg. no. 2610 2004-01-15 |

|
Patient information website. Indicates a website where a patient can obtain additional information on the medical product |
ISO 15223-1:2020. 5.7.4 ISO 7000-3705 2019-10-18 |

|
Medical Device. Indicates the item is a medical device |
ISO 15223-1:2020. 5.7.7 |
|
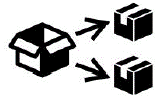
Repackaging To identify that a modification to the original medical device packaging configuration has occurred |
ISO 15223-1:2020. 5.7.9 ISO 7000-3727 2019-11-01 |

|
Unique Device Identifier. Indicates a carrier that contains Unique Device Identifier information |
ISO 15223-1:2020. 5.7.10 |

|
UK Conformity Assessed Marking Indicates conformity with local laws and regulations in Great Britain (England, Wales and Scotland) |
UK MDR 2002 |

|
Conformitè Europëenne / European Conformity Indicates conformity with local laws and regulations within the European Economic Area |